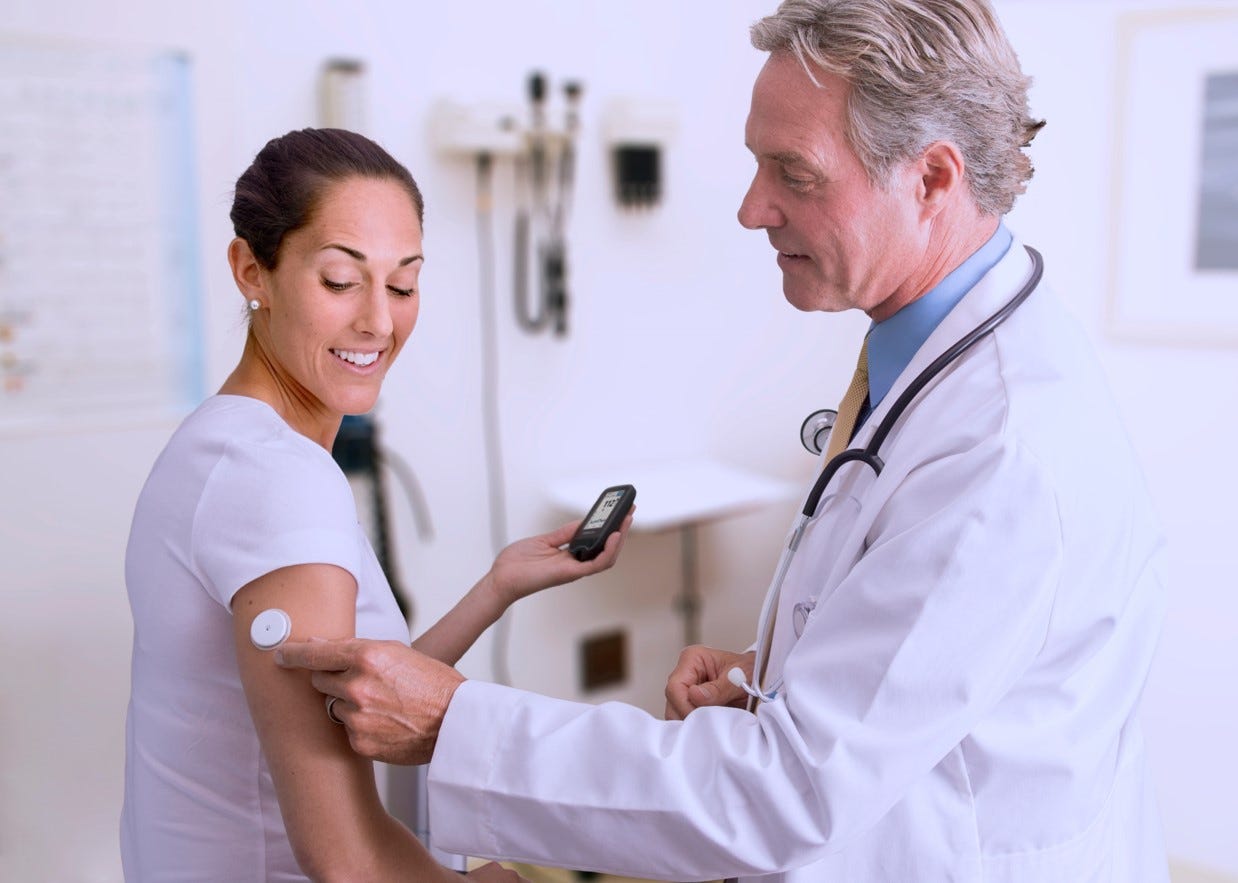

TRUST THE #1 SENSOR-BASED
GLUCOSE MONITORING
SYSTEM IN THE WORLD1
Published in >1000 clinical studies2, and the most recommended brand with over 7 million1 users worldwide.
FreeStyle Libre |
|
Brand D |
|
Accuracy  |
FreeStyle Libre systems meet U.S. FDA’s iCGM3 standards. | Meet U.S. FDA’s iCGM3 standards. | |
| Wear Duration |
14 days4 | 10 days5 | |
Cost / Day  |
$7.366 | $9.597 | |
Sensor Size |
35 x 5 mm4 | 46 x 30 x 15 mm8 |
REFERENCES:
-
Data on file, Abbott Diabetes Care, Inc. Based on the number of users worldwide for the FreeStyle Libre portfolio compared to the number of users for other leading personal use sensor-based glucose monitoring systems.
-
Peer-reviewed abstracts and publications collected from January 2011 – January 2023.
-
iCGM is a set of special controls that outlines the requirements for assuring iCGM devices’ accuracy, reliability and clinical relevance as well as describe the type of studies and data required to demonstrate acceptable iCGM performance. Food and Drug Administration (FDA). Classification of the integrated continuous glucose monitoring system. Federal register February 18. 2022. Federal Register: Medical Devices; Clinical Chemistry and Clinical Toxicology Devices; Classification of the Integrated Continuous Glucose Monitoring System (Accessed Dec 2024). iCGM is a US only classification and regulation.
-
FreeStyle Libre User Manual
-
Brand D CGM User Guide (Data on File).
-
Based on website price of $103.10/sensor.
-
Based on website (https://shopee.sg/brandD) of $95.90 for 1 sensor + 1 transmitter (Accessed Dec 2024). (Data on File).
- Transmitter and sensor size, Brand D Tech Sheet (Accessed Jan 2025). (Data on File).
© 2025 Abbott. All Rights Reserved. The sensor housing, FreeStyle, Libre, and related brand marks are trademarks of Abbott. Other trademarks are the property of their respective owners. ADC-105760 v2.0






